Where innovation meets efficiency in the world of quality management. Greenlight Guru empower companies with cutting-edge software and comprehensive processes
and user experience, facilitating accelerated growth, enhanced efficiency, and minimised risk throughout the entire product lifecycle.

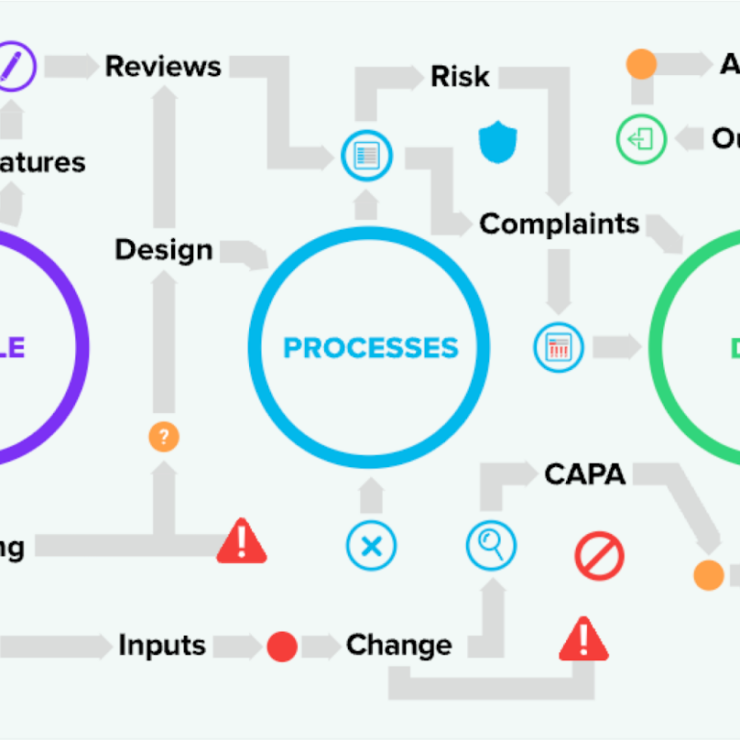
The possibilities are endless with tracking quality events, trace design controls to risk, ensure compliance, and more. Greenlight Guru QMS software not only accelerates bringing medical devices to market, but also ensures their sustained success. By choosing a Greenlight Guru system, creates ease of a unified team under one interconnected quality ecosystem with the #1 eQMS for medical device companies.
We have a powerful and experienced team to
implement Greenlight Guru systems according to regulatory requirements specific to each market.
We align company’s processes with
system operations.
We have a validation team that helps the customer to validate the configured system according to their requirements and computer system validation policies, following the complete system lifecycle and reducing the time required for Go-Live.
Rephine can offer ad hoc training to ensure that the overall processes are efficient and compliant with current expectations. This will ensure that your team gets suitable experience and knowledge to maximise the advantages of using the software to elevate your quality and regulatory processes to proficiency levels.
Our Greenlight Guru team of experts can provide maintenance and enhancement assistance to help you maximising the success of your Quality Management System, implementing new processes, improving the existing ones or rolling them out in new sites, divisions or business areas.
Shift from paper to cloud-based platforms for accurate management of Medical Device documentation. Embrace a comprehensive end-to-end approach through Rephine and Greenlight Guru collaboration to eliminate silos, ensure high-standard compliance, and streamline daily activities. Choose a tailored Document Management plan for seamless navigation of regulatory complexities and confident delivery of innovative Medical Devices.
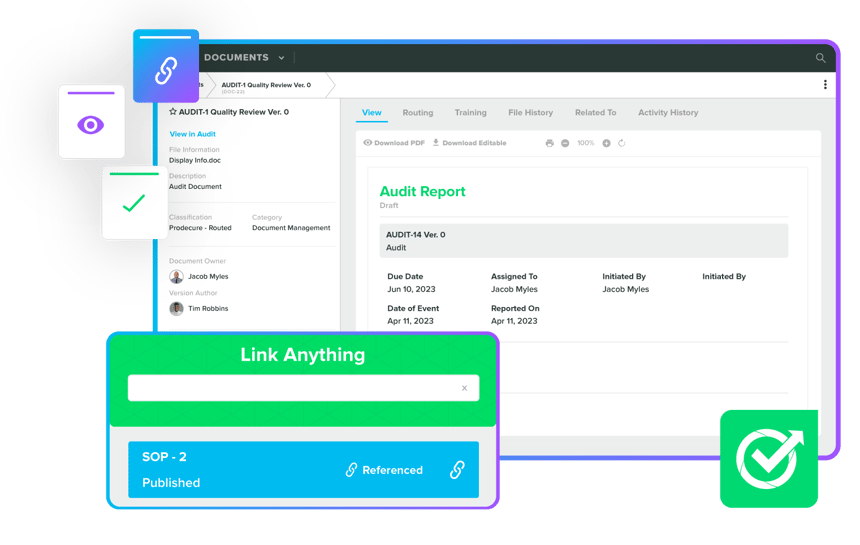
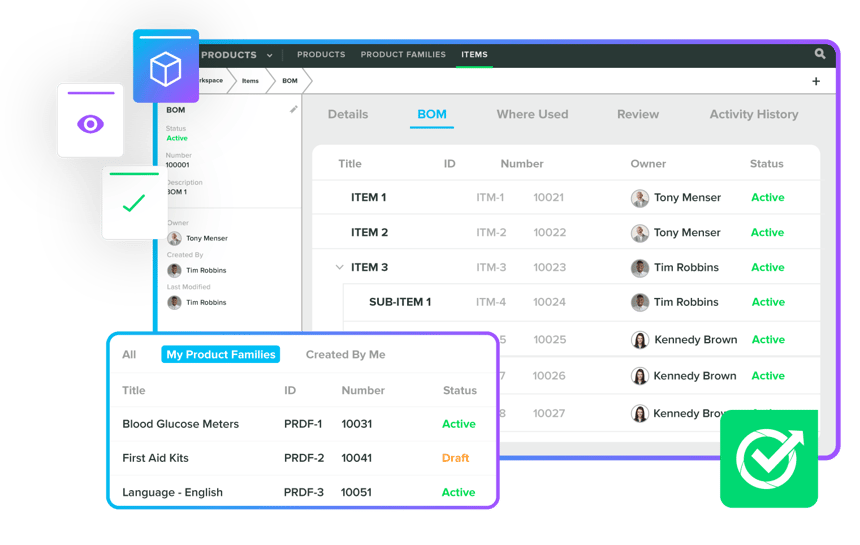
Enhance timelines and efficiency with Product Management & Design Controls. Our Greenlight Guru experts implement compliant and organised records by automating tasks like design control matrices, order changes, BOMs, and DHFs. We ensure comprehensive BOM control, multi-level structures, and organised product families, ensuring successful collaborations, high-standard compliance, and regulatory submissions for ISO certifications.
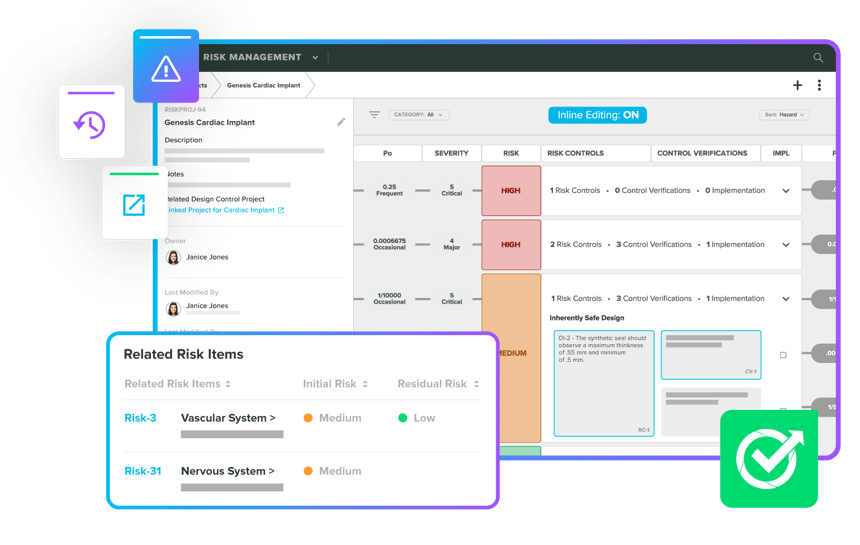
Leverage the expertise of our Greenlight Guru team for a quality and regulatory perspective on Risk Management. Enjoy seamless integration with full traceability in your QMS. Effortlessly integrate risk-based thinking across your device ecosystem, ensuring compliance with ISO 14971:2019 and ISO 13485:2016. Whether creating Risk Management Files or managing post-market risks, our experts have you fully covered.
Integrate the Quality Process & CAPA Management system, specificially designed to efficiently handle MedTech CAPAs. Collect data, documents, design components, and quality events within your QMS, ensuring proactive planning with traceable and compliant workflows for effective issue correction and prevention. Automate your CAPA process to simplify it, and tailor custom workflows that align with your specific needs.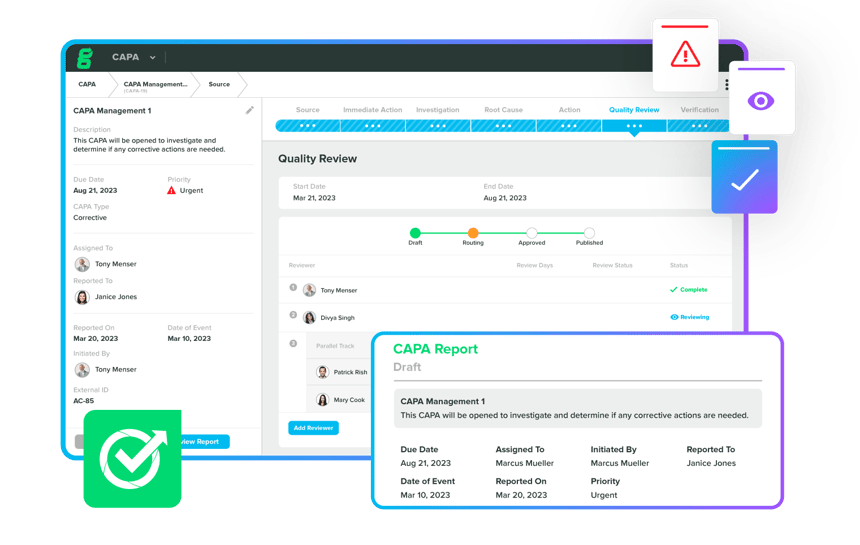
Discover more from the official launch below
By extending our services of implementation and improvement of Quality Management Systems (QMS) for Medical Devices, to the
automation and optimisation of key quality processes, our commitment is to ensure we are implementing the best MDQMS platforms
available today.
As a result, a clear benefit for our clients implementing Greenlight Guru Quality:





Our Medical Device experts can provide ongoing assistance with customisable QMS systems, regulatory consultancy, and more, to help with your QA journey.
Discover our other Medical Device Services and how are experts can help you.
We provide extensive GMP consulting services to help keep our clients ahead of the needs and expectations of regulators.
Explore our extensive GMP audit library to see the range and scope of live reports we have in stock, join a live audit, or commission a bespoke audit
Maintain high standards of life sciences manufacturing supplier qualifications and GMP auditing within the supply chain through our expertise
Discover how we can help your product reach to market, fully and demonstrably complying with the latest GxP standards
From data integrity to implementing new systems, our experienced team with a digital mindset, can lead you to transformative achievements
REPHINE CHINA
REPHINE INDIA
Sign up to our newsletter to get the latest news about Rephine and industry news.