
Nowadays, many companies must face new challenges and situations that arise from new business opportunities: the development of a new product, the opening of new markets in new countries or geographical areas, agreements with international companies for a project on a global scale… This results in the need to comply with new or different regulations, not previously considered.
Opening to other markets, either in Europe, USA, Asia-Pacific, the Middle East or Latin America, requires the need of compliance to high standards of cGMP and the success in the subsequent inspections, a pre-requisite for the approval of new activities or products.
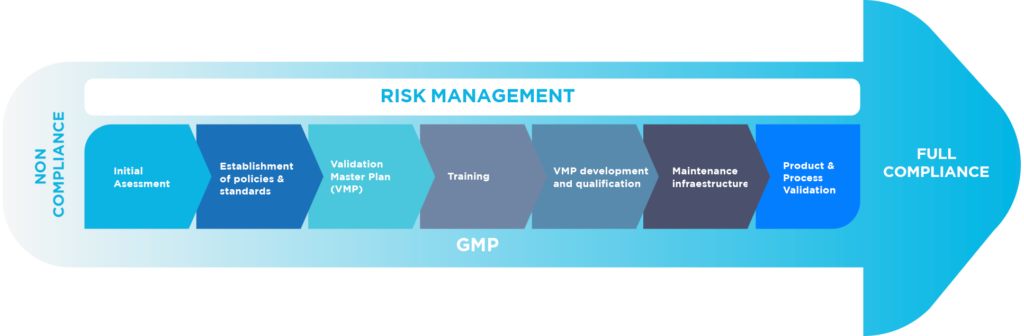


.

We provide extensive GMP consulting services to help keep our clients ahead of the needs and expectations of regulators.
Explore our extensive GMP audit library to see the range and scope of live reports we have in stock, join a live audit, or commission a bespoke audit
Maintain high standards of life sciences manufacturing supplier qualifications and GMP auditing within the supply chain through our expertise
Discover how we can help your product reach to market, fully and demonstrably complying with the latest GxP standards
From data integrity to implementing new systems, our experienced team with a digital mindset, can lead you to transformative achievements
REPHINE CHINA
REPHINE INDIA
After a rigorous process for becoming certified by Sparta, we have a powerful and experienced team to implement TrackWise systems according of each client’s requirements. These requirements are captured through different workshops and subsequent prototypes are made.
After a rigorous process for becoming certified by Sparta, we have a powerful and experienced team to implement TrackWise systems according of each client’s requirements. These requirements are captured through different workshops and subsequent prototypes are made.
Sign up to our newsletter to get the latest news about Rephine and industry news.