In this environment, the distribution and transport of medicines and active ingredients (APIs) for human use are key supply chain activities that must be controlled in order to maintain the quality and integrity of the medicines, as set forth in the European regulations, 2011/62/EU and related Guides 2013/C 343/01 and 2015/C 95/01, as well as current FDA initiatives and expectations.
How to identify all the actors involved in the supply chain?
What controls should be established along the supply chain?
Who should have a GDP certificate for the distribution or transport of medicines?
What is a GDP certificate and who can issue it?
How responsibilities along the supply chain should be defined?


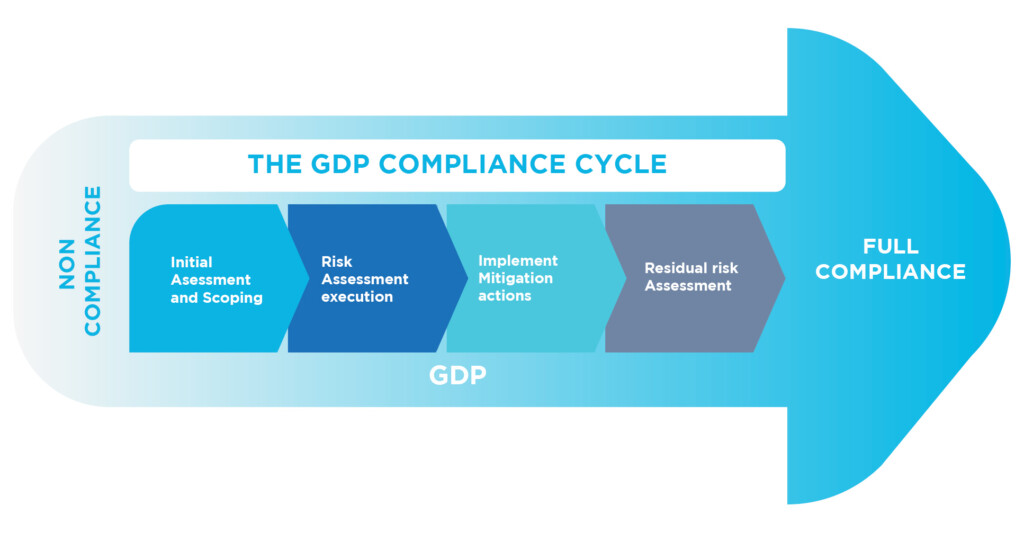
We provide extensive GMP consulting services to help keep our clients ahead of the needs and expectations of regulators.
Explore our extensive GMP audit library to see the range and scope of live reports we have in stock, join a live audit, or commission a bespoke audit
Maintain high standards of life sciences manufacturing supplier qualifications and GMP auditing within the supply chain through our expertise
Discover how we can help your product reach to market, fully and demonstrably complying with the latest GxP standards
From data integrity to implementing new systems, our experienced team with a digital mindset, can lead you to transformative achievements
REPHINE CHINA
REPHINE INDIA
Sign up to our newsletter to get the latest news about Rephine and industry news.