Tackling Regulatory Challenges: Your Guide to DMF Submission in China
Navigating China’s pharmaceutical market is no easy feat, especially when it comes to API and excipient registration. To sell in China, companies must submit a Drug Master File (DMF) to the National Medical Products Administration (NMPA) — a process known for its regulatory complexities, administrative demands, and language barriers.
Understanding the DMF Process in China
A DMF submission involves more than just sending over a technical file. Foreign manufacturers must appoint a local agent — either a subsidiary or a registered representative — to handle the submission and all related communications with the Centre for Drug Evaluation (CDE) and NMPA.
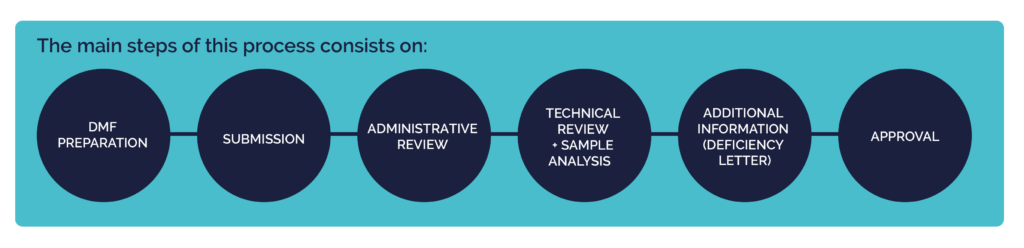
The main steps include:
- Reviewing the existing DMF for compliance with Chinese guidelines.
- Translating all documentation into Chinese.
- Managing administrative tasks, including registering as an official agent and preparing complete document packages.
- Handling deficiency letters from the CDE and ensuring timely responses.
- Coordinating shipment of required samples and ensuring all additional data requests are met.
Without local expertise, delays, misunderstandings, and compliance failures can easily derail the process.

Why Rephine?
Rephine bridges the gap between international pharmaceutical companies and the Chinese market. With offices in Europe and Shanghai, we combine global standards with local know-how, ensuring the process runs smoothly while safeguarding information confidentiality.
Our services include:
- Full regulatory and technical assistance for DMF submissions.
- Acting as the official agent for administrative management.
- Handling all communication with CDE and NMPA, including translations.
- Ensuring compliance with evolving Chinese regulations.
Rephine’s Proven DMF Submission Process
Real-World Success: Overcoming Regulatory Hurdles — A Case Study
One of Rephine’s international API manufacturing clients faced significant challenges with their DMF submission in China. From language barriers to regulatory nuances, the process felt daunting.
Rephine provided end-to-end support — conducting a detailed compliance review of the existing DMF, ensuring alignment with Chinese guidelines and the Chinese Pharmacopoeia. They managed translations, registered as the official agent with the NMPA, and handled all administrative processes, from documentation submission to deficiency letter responses. Rephine’s fast, coordinated efforts led to a successful, timely DMF approval, enabling market access.
Panreac Química, a leading global manufacturer of excipients, praised Rephine’s expertise:
“Rephine’s expertise, professionalism, and deep understanding of regulatory requirements were key to our success.” — Panreac Química

